Solid State Lithium Metal Batteries
We are working on solid block copolymers and glass-polymer composites that selectively transport lithium ions. These materials are used to build all-solid rechargeable lithium batteries. We use characterization techniques such as AC impedance, NMR, electron microscopy, X-ray absorption and scattering, and hard X-ray microtomography. These tools allow us to understand the morphology, ion transport properties, and failure mechanisms of the electrolyte.
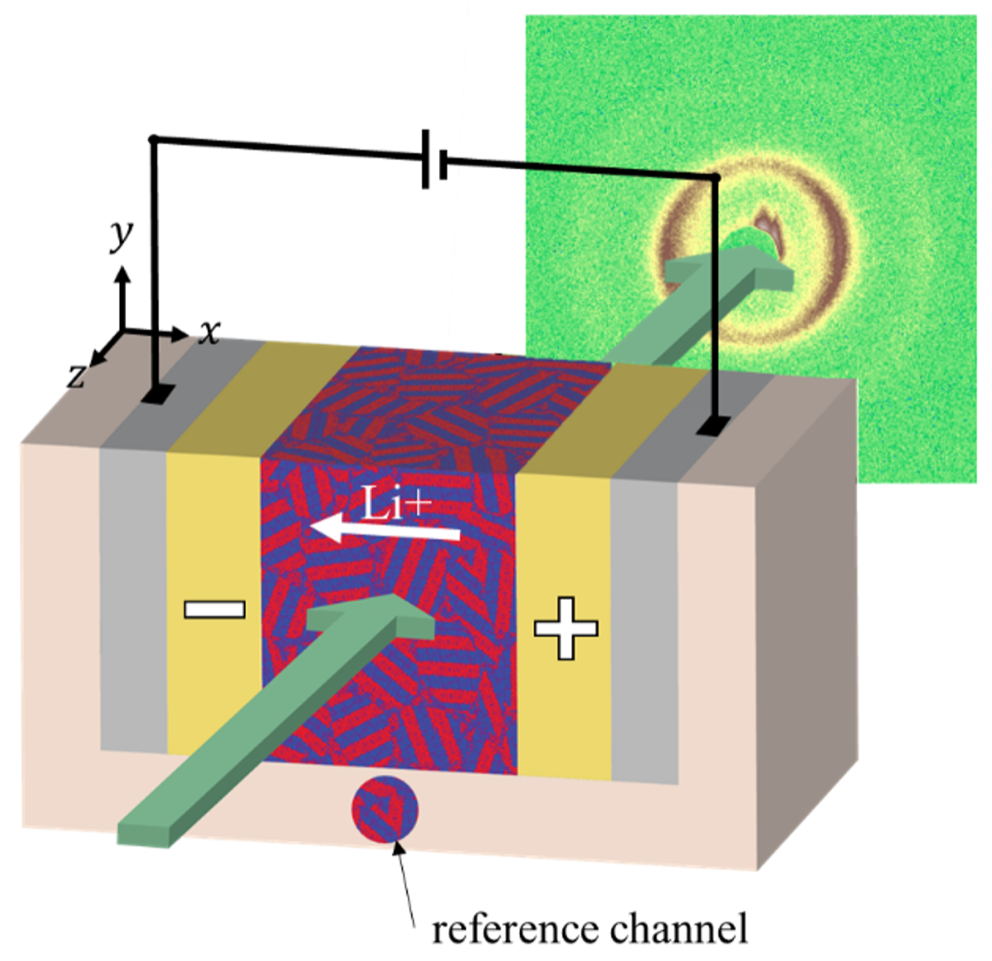
in situ X-ray Scattering
As current is passed through an electrochemical cell, a concentration gradient builds up, with the salt concentration increasing near one electrode and decreasing near the other. In block copolymer electrolytes, the salt concentration affects the local microstructure in complex ways. For example, as salt concentration increases, salt-containing domains swell, potentially applying strain to neighboring domains. In this project, we use a custom-build electrochemical cell setup which allows us to use small-angle x-ray scattering to study morphological changes as a function of position over time during application of a potential. These morphological changes can have significant effects on the electrochemical performance of the cell. As of Fall 2021, we are recruiting a Berkeley first-year graduate student to work on this project.
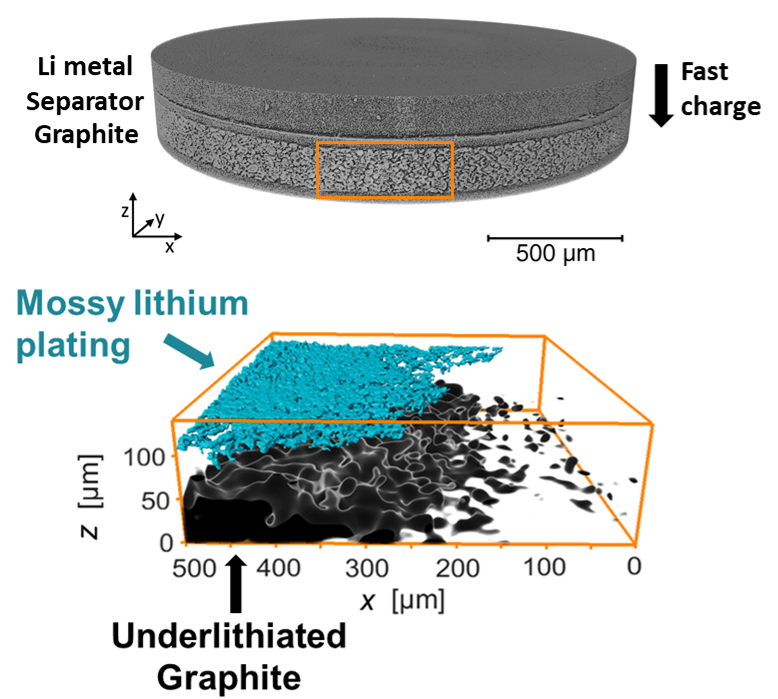
in situ X-ray Tomography
A barrier to fast charging lithium-ion batteries is the deleterious side reaction of lithium plating on graphite, reducing cell capacity and causing safety concerns. Fast charging conditions also cause uneven lithiation of the graphite electrode. We use hard X-ray microtomography to study batteries in situ to find lithium plating and track lithiation heterogeneities in 3D. Correlating these bottlenecks allows us to discover pheneomena such as the "shadow effect", where lithium plating at the front of the graphite electrode blocks transport to the back. As of Fall 2021, we are recruiting a Berkeley first-year graduate student to work on this project.
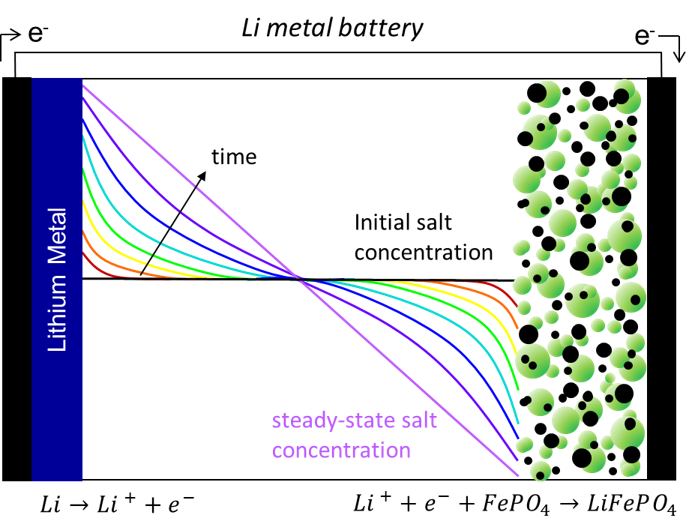
Electrochemistry: Ion Transport Properties
Ion transport in a binary electrolyte is characterized by three transport parameters: conductivity (κ), cation transference number (𝑡+0), and the salt diffusion coefficient (D). When these three parameters are known for an electrolyte as a function of salt concentration, ion transport can be modeled to make useful predictions such as the salt concentration as a function of distance between the electrodes (shown left) or the maximum current density that can be passed through the cell. This maximum current density, known as the limiting current, is essential for comparing electrolytes and understanding the conditions at which an electrolyte will fail. In addition to using AC impedance to measure the three transport parameters and the limiting current, our group uses spectroscopic techniques such as Raman microscopy, NMR, XPCS, and SAXS to make real time observations of salt concentration gradients that develop within the electrolyte in response to an applied current.
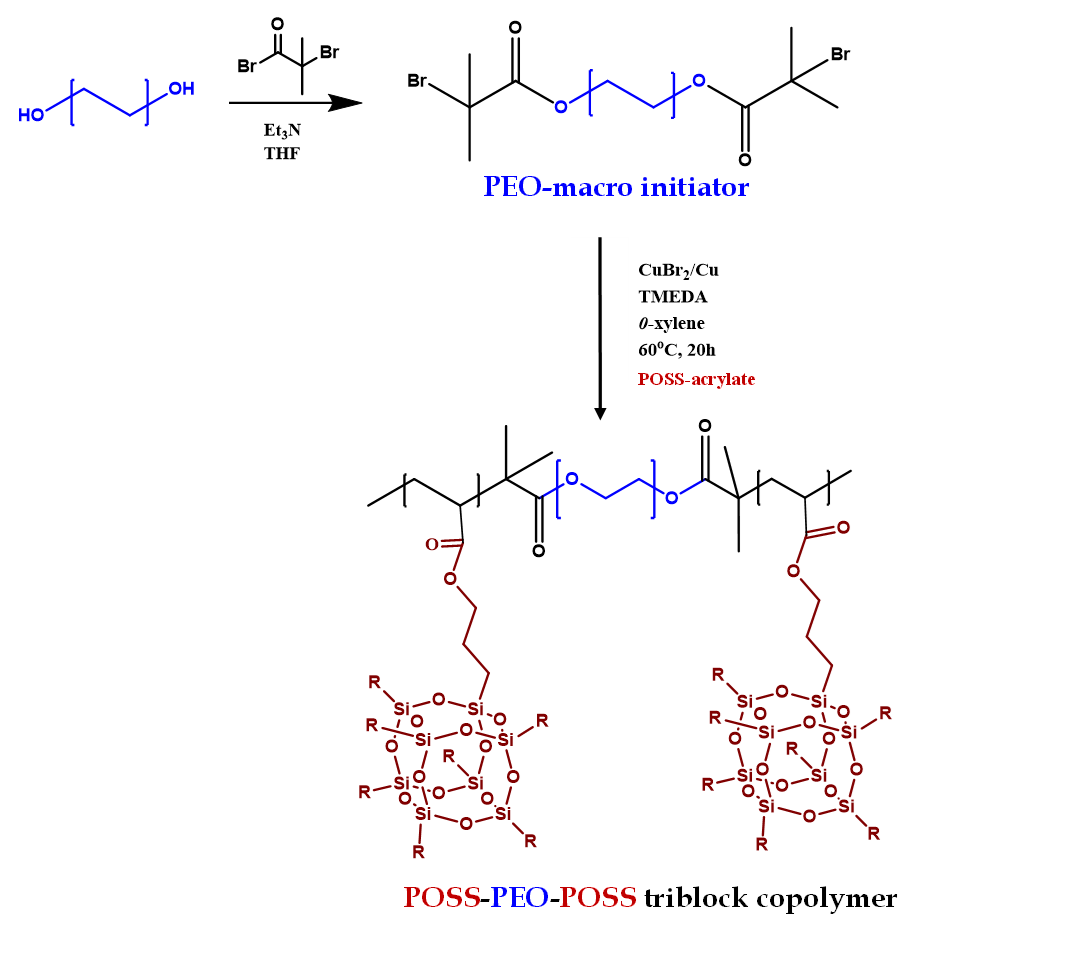
Design and synthesis of block copolymer electrolytes
We are working on developing solid block copolymers and composite-polymer electrolytes that can selectively transport lithium ions. We carry out various polymerization techniques for example anionic polymerization, condensation polymerization, nitroxide mediated polymerization (NMP), reversible deactivation radical polymerization (RDRP) etc. for the synthesis. We utilize neuclear magnetic resonance spectroscopy (NMR), gel permeation chromatography (GPC), Raman spectroscopy, electron microscopy, X-ray scattering (SAXS/WAXS) techniques to characterize the polymers.
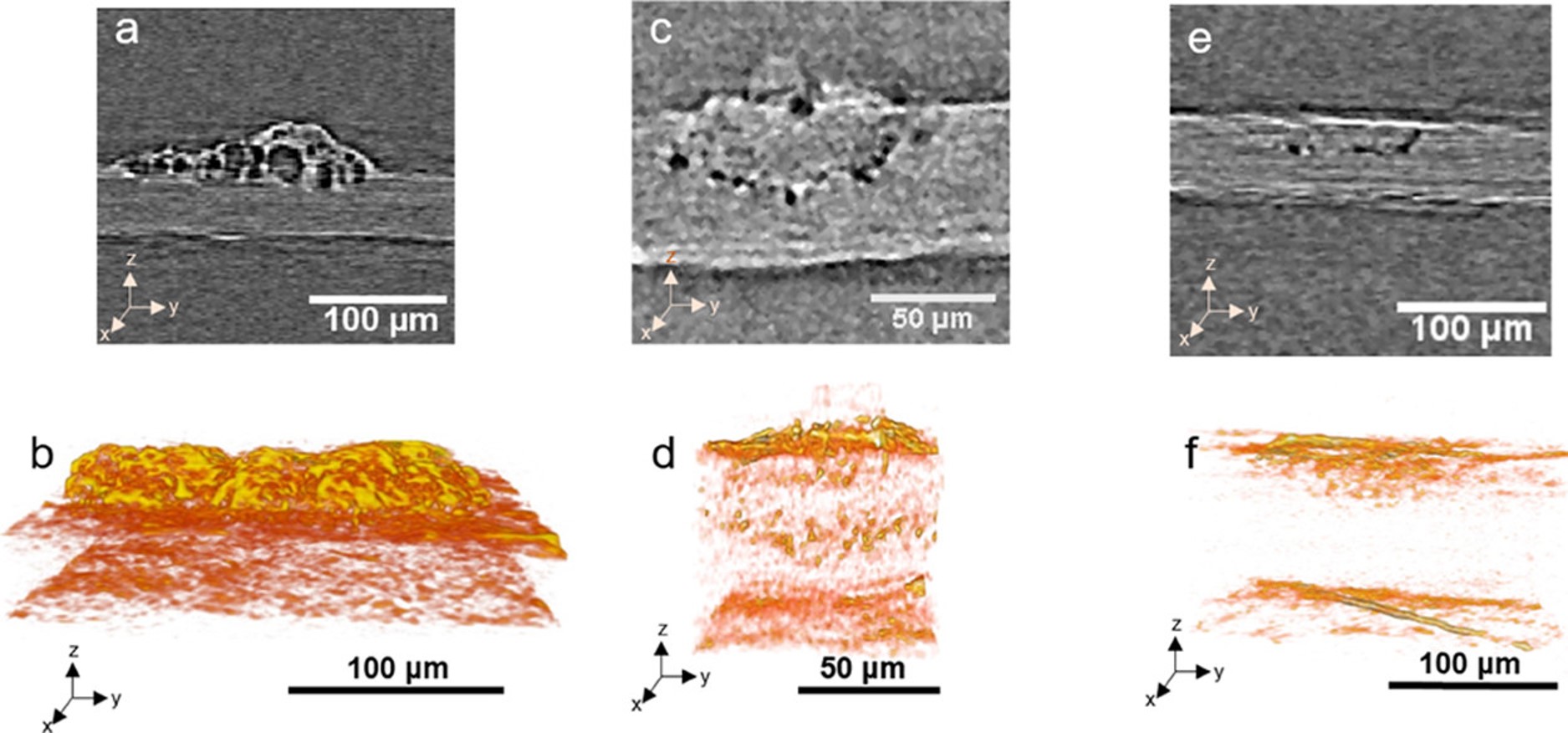
Effect of mechanical strength of electrolytes on dendrite formation
One of the major challenges in implementing lithiumiion batteries is irregular electrodeposition of lithium metal during cycling. These irregular growth are often known as dendrites. Mechanically rigid polymers can suppress the growth of dendrites. Polymers are viscoelastic materials. The mechanical properties, such as storage-loss modulus and yield stress of the block copolymers are measured using rheometry. We try to correlate the mechanical properties of the polymers to the performance of the electrolytes and the cell failure through dendrite formation.
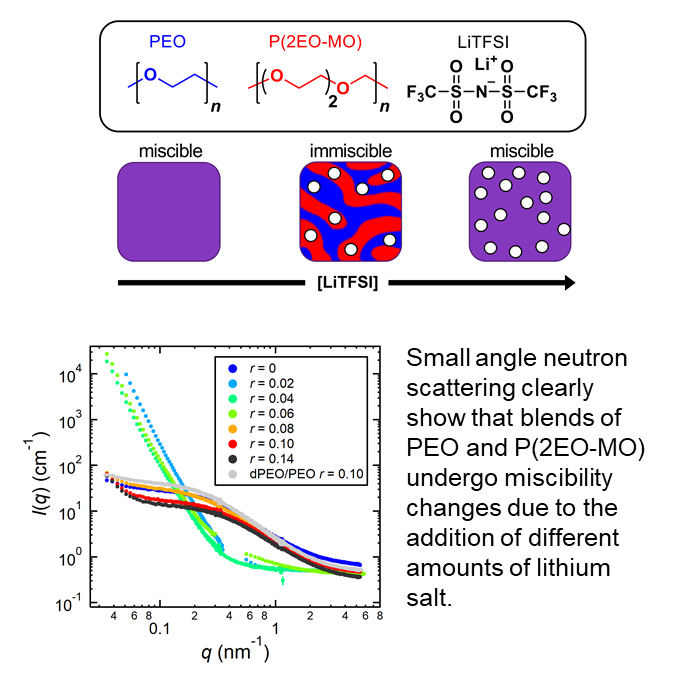
Polymer blends and neutron scattering
Modern lithium-ion batteries use blends of organic solvents as an electrolyte. Replacing this flammable electrolyte with inherently safer blends of nonvolatile polymers is challenging because polymers rarely mix. We show, via small angle neutron scattering, that blends of poly(ethylene oxide) (PEO) and poly(1,3,6-trioxocane) (P(2EO-MO)) with a lithium salt can result in homogeneous mixtures that can be used for electrolytic applications.
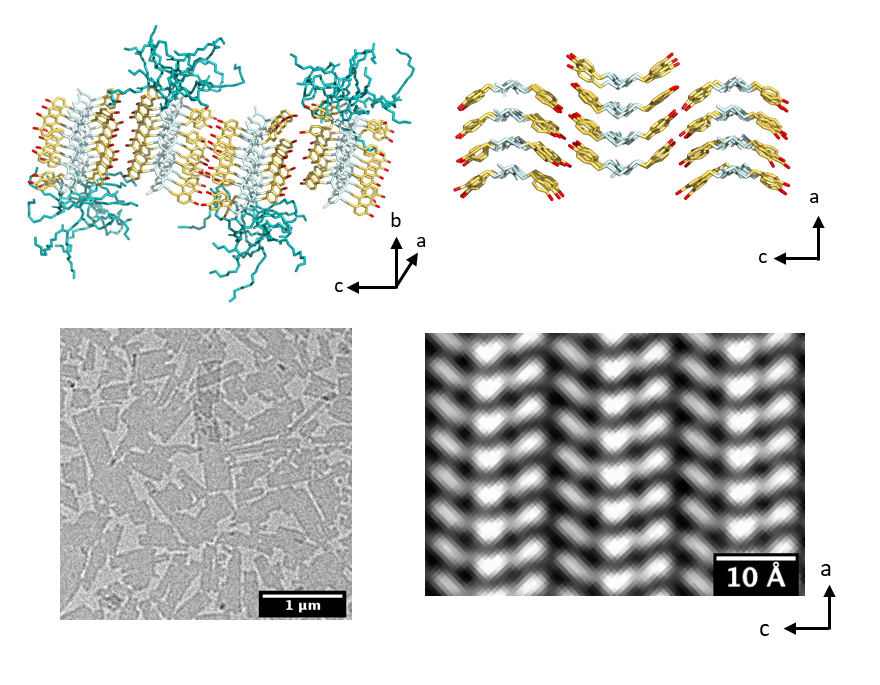
Cryo-electron microscopy
We use cryo-EM to obtain atomic-scale images of self-assembled peptoid nanostructures. We collaborate with the Soft Matter Electron Microscopy Program at LBL for synthesis, characterization (X-ray scattering, DSC, and AFM), and simulations of the peptoid nanostructures. By combining atomic-scale information from experiments and simulations, we want to learn more about the molecular self-assembly process in these materials in order to engineer functional nanomaterials.
